At Avik Pharmaceutical Ltd, our philosophy behind producing Calcitriol API extends far beyond routine synthesis. We view it as a molecule that demands precision, protection, and scientific rigor at every step — and our processes are engineered to meet that responsibility with unmatched integrity.
Why Calcitriol Is Critical
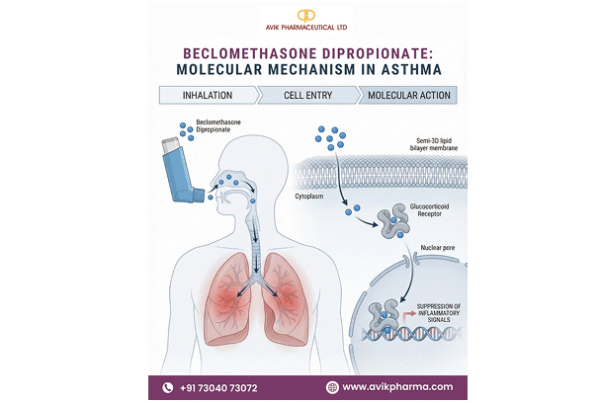
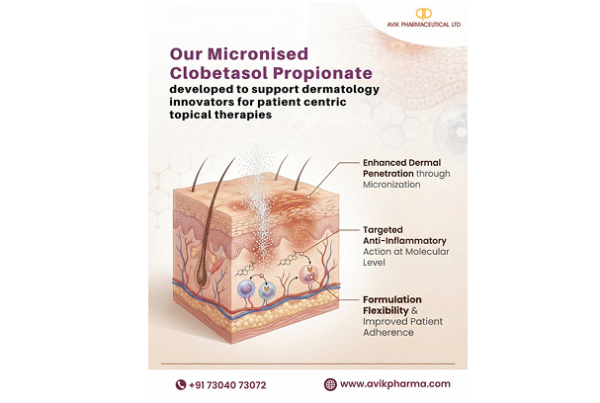
Calcitriol, the active hormonal form of Vitamin D, exhibits up to 1,000 times greater receptor-binding potency compared to standard Vitamin D2 or D3. This elevates it from a nutritional supplement component to a powerful therapeutic agent. Its applications in treating hypocalcemia, renal osteodystrophy, osteoporosis, and rickets make reliability and purity absolutely crucial for patient outcomes.
Why Calcitriol Is Critical
Our manufacturing ecosystem is designed around protecting this highly sensitive molecule. From temperature-controlled handling of KSMs to the final API, each stage is carefully monitored. We employ monochromatic light during processing to prevent photodegradation and use advanced photochemical conversion techniques for superior precision. Closed-loop purification, inert gas purging, and amber-glass vial packing ensure safety and stability throughout transit.
All batches are produced under Class D (1,00,000) AHU conditions using AR & ER grade solvents, supported by rigorous HPLC-based in-process monitoring and validated analytical methods.
With dedicated infrastructure and specialized expertise, Avik is equipped to deliver both standard and pharmacopeia-specific grades for regulated markets.
Let’s partner to ensure a dependable, high-purity Calcitriol supply that meets global quality expectations.