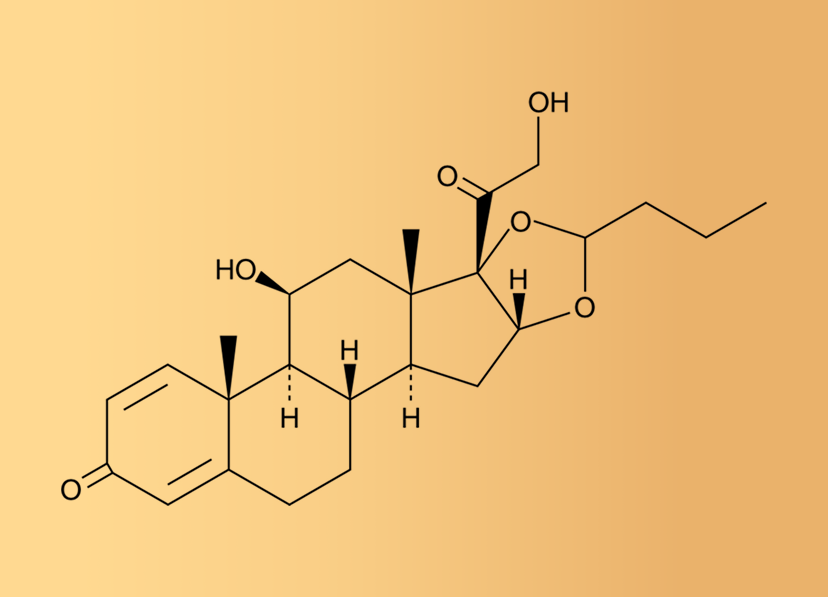

Budesonide
Commercial
Anti-inflammatory
IP/ BP /EP
5133 – 22 – 3
CEP No. R1-CEP 2014 – 177-Rev 00
ANVISA, KFDA, CDE-NMPA, PATENT GRANTED
WO 2018/033939 A1
IN295893
Application - suspension, capsule, tablet, inhaler, injectables
Drug Description
Budesonide extended-release capsules are indicated for the treatment and maintenance of mild to moderate Crohn’s disease. Various inhaled budesonide products are indicated for prophylactic therapy in asthma and to reduce exacerbations of COPD. A budesonide nasal spray is available over the counter for symptoms of hay fever and upper respiratory allergies.







